Audio added September 22, 2020.
H2(1/4) gas was collected from a SunCell® operated at a cell pressure of 10-20 Torr over 100s using a valved microchamber connected to the vacuum line and cooled to 10.5 K by a cryopump system (Helix Corp., CTI-Cryogenics Model SC compressor; TRI-Research Model T-2000D-IEEE controller; Helix Corp., CTI-Cryogenics model 22 cryodyne). The SunCell® comprised a Type 347 stainless steel (SS) cylindrical tube measuring 7.3 cm ID, 19.7 cm in height, and 0.635 cm thick with 3.17 mm thick boron nitride (99%) liner and incorporating a 0.9 kg internal mass of liquid gallium wherein the gas flow rates were 2500 sccm H2/50 sccm O2, and the ignition current was 1500 A. Argon and trace oxygen were flowed before the reaction was initiated to serve as a solvent for hydrino gas H2(1/4).
The liquefied gas was warmed to room temperature to achieve 23 Torr chamber pressure and was injected into an HP 5890 Series II gas chromatograph with a capillary column (Agilent molecular sieve 5 Å, (50 m x 0.32, df = 30 μm) at 303 K (30 °C), argon carrier gas, and a thermal conductivity detector (TCD) at 60 °C.
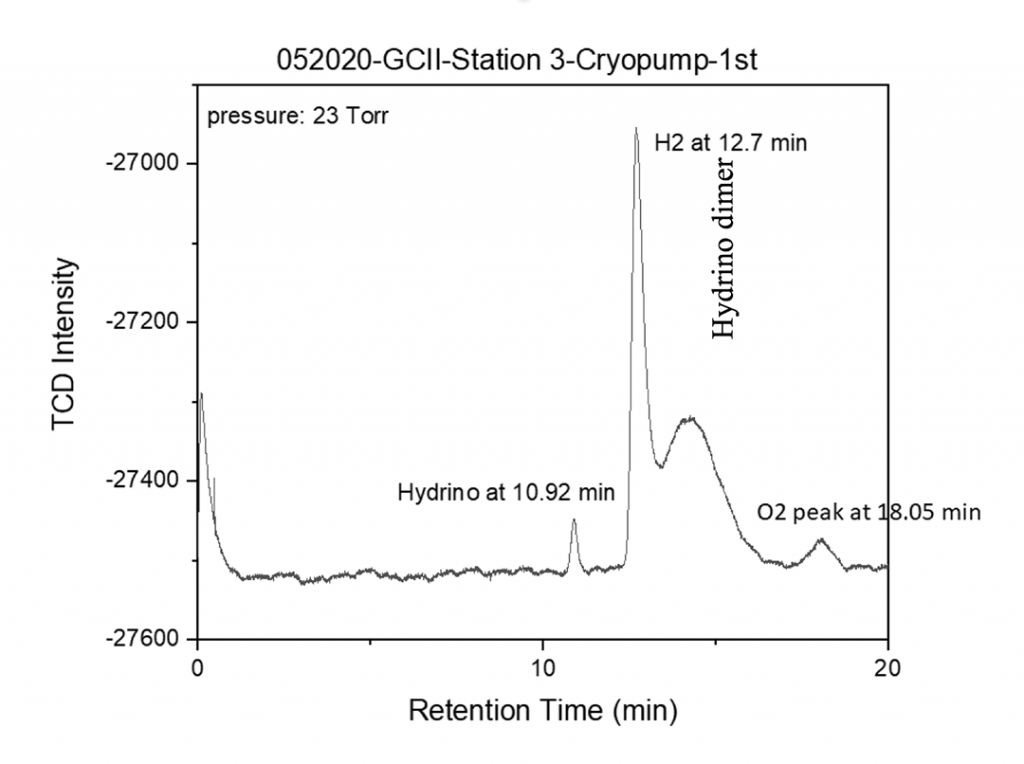
- H2(1/4) was observed at 10.92 minutes, [H2(1/4)]2 was observed as a broad peak at 15 minutes, oxygen was observed at 18.05 minutes, and hydrogen that co-condensed with H2(1/4) gas was observed at 12.7 minutes.
- Hydrogen condensed under pressure and temperature conditions that violate the Clausius Clapeyron equation due to the raising of the H2 liquefaction temperature by co-condensation with H2(1/4).
